Which Element Commonly Has Only a Proton as Its Nucleus
A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus for example carbon-13 with 6 protons and 7 neutrons. When 226 Ra emits an α particle 4 He two protons and two neutrons are carried away Fig.
The smallest possible amount of matter which still retains its identity as a chemical element consisting of a nucleus surrounded by electrons.
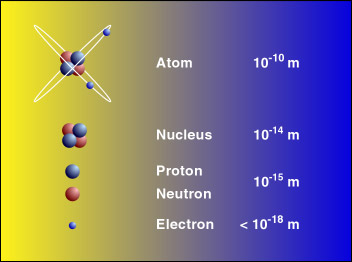
. The product of radium α decay the daughter is a different element. The role of the element in humans animals and plants. We accept only Visa MasterCard American Express and Discover for online orders.
Therefore the residual nucleus has 86 protons and 136 neutrons. The description of the element in its natural form. Hydrogen for example has one proton and one electron with no neutrons.
For example the radium nucleus Z 88 A 226 has 88 protons and 138 neutrons. Neutrons are uncharged particles found within the nucleus. You will get a personal manager and a discount.
Type of paper needed. Isotope definition any of two or more forms of a chemical element having the same number of protons in the nucleus or the same atomic number but having different numbers of neutrons in the nucleus or different atomic weights. Your personal details remain confidential and wont be disclosed to the writer or other parties.
FlexBook Platform FlexBook FlexLet and FlexCard are registered trademarks of CK-12 Foundation. New to Coursework Hero. Where the element is most commonly found in nature and how it is sourced commercially.
Proton definition a positively charged elementary particle that is a fundamental constituent of all atomic nuclei. The shell closest to the nucleus can hold two electrons. Sign up Save Calculate the price of your order.
The nuclide concept referring to individual nuclear species emphasizes nuclear properties over chemical properties whereas the isotope concept grouping all atoms of each element emphasizes. Electrons usually remain a constant distance from the nucleus in precise shells. The number of protons determines which element it is.
Every atom of carbon has six protons six electrons and six neutrons. The neutron is a subatomic particle symbol n or n 0 which has a neutral not positive or negative charge and a mass slightly greater than that of a protonProtons and neutrons constitute the nuclei of atomsSince protons and neutrons behave similarly within the nucleus and each has a mass of approximately one atomic mass unit they are both referred to as nucleons. It is the lightest and most stable baryon having a charge equal in magnitude to that of the electron a spin of ½ and a mass of 1673 10-27 kg.
The image is based on the iconic atomic model first proposed by Niels Bohr in 1913. There are 275 isotopes of the 81 stable elements in addition to over 800 radioactive isotopes and every element has known isotopic forms. Each electron has a negative charge -1 equal to the positive charge of a proton 1.
Eas111 Chapter 2 Atomic Structure
2 1 The Building Blocks Of Molecules Concepts Of Biology 1st Canadian Edition
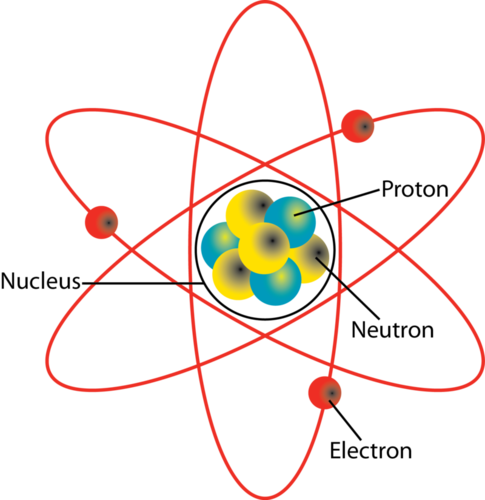
What Two Particles Are Found In The Nucleus Of An Atom Socratic

Comments
Post a Comment