How Many Valence Electrons Do Transition Metals Have
Indeed the most stable valency for all the elements is three. Why does scandium have 9 electrons in the third shell.
288 right it readily accepts the electron given by the sodium atom.
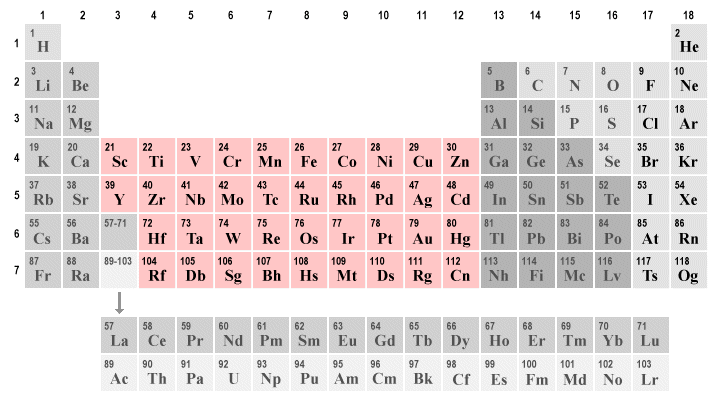
. Most have two with several notable exceptions. The total number of valence electrons is 5611. Valence electrons in Fluorine F 7.
The answer is b boron aluminum has 3 valence electrons magnesium has 2 boron has 3 silicon has 6 scandium and vanadium are both transition metals with 2s4 and 9 and 11 n 3 electrons. An atom consisting of a closed shell of valence electrons will usually be chemically inert. Most transition metals have 2 valence electrons.
Atoms of group 18 elements have eight valence. 3 question In general how many valence electrons do transition metals have. Therefore the valence electrons of cobalt Co are nine.
Elements in the d block are transition elements and each posses one or two valence electrons in their respective s orbitals. A striking feature of the valencies is that they vary much less than they do for transition elements and also for the actinides. Valence electrons are the sum total of all the electrons in the highest energy level principal quantum number n.
They are unlikely to gain or lose electrons or to share electrons with other atoms. They are unlikely to gain or lose electrons or to share electrons with other atoms. Valence electrons in Oxygen O 6.
Most transition metals have an electron configuration that is ns2 n1d so those ns2 electrons are the valence electrons. Group 2 period 3 blocks. 4 rows Most transition metals have 2 valence electrons.
Valence electrons are the sum total of all the electrons in the highest energy level principal quantum number n. Therefore no matter how electrons are shared between the nitrogen and oxygen atoms there is no way for nitrogen to have an octet. It will have seven electrons assuming that the oxygen atom does satisfy the octet.
Noble Gases The Group 18 elements are the noble gases. For the main group elements the valence electron exists only in the outermost electron shell. Most transition metals have 2 valence electrons.
Atoms of the noble gases have 8 valence electrons except for helium which has 2. To find the number of valence electrons for Transition Metals we need to look at its electron configuration. A closed shell of valence electrons ie eight electrons in the outermost shell of an atom will make the atom chemically inert.
Elements may steal an electron from the outermost s block and relocate it to the d block in order to reach a filled or half-filled 5 of 10 electrons state in the d block. Valence electrons in Silicon Si 4. Valence electrons in Phosphorus.
The electron configuration of manganese shows that the last shell of manganese has two electrons and the d-orbital has a total of five electrons 3d 5. Contains inner transition metals lanthanides and actinides Group 17 period5 blockp. Most transition metals have 2 valence electrons.
How Many Valence Electrons Does Potassium Have. Valence electrons in Neon Ne 8. Where n 3 electrons fill last definitely not similar to aluminum generally the easy way to answer this is anything in the same column has the same valence electrons.
Valence electrons in Magnesium Mg 2. Valence electrons are the sum total of all the electrons in the highest energy level principal quantum number n. In this valency the lanthanides live up to their reputation of being very similar.
Atoms of the noble gases have 8 valence electrons except for helium which has 2. Valence are highest n only s and p sublevel What ion is likely to form with transition metals. For the transition element the valence electrons have to be determined by adding the total electrons of the d-orbital to the electrons in the last orbit of the atom.
The last shell of cobalt Co has two 4s 2 electrons and the d-orbital has a total of seven electrons 3d 7. For a transition metal a valence electron can exist in the inner shells also. For the transition element the valence electron has to be determined by adding the total electrons of the d-orbital to the electron in the last shell of the atom.
This is necessary because for Transition Metals. 119 rows Valence electrons in Nitrogen N 5. Valence electrons are the sum total of all the electrons in the highest energy level principal quantum number n.
Most transition metals have an electron configuration that is ns2 n-1d so those ns2 electrons are the valence electrons. Determine group period and block from the given electron configuration. Valence electrons in Aluminum Al 3.
Valence electrons in Sodium Na 1. Atoms with 8 valence electrons or 2 in the case of helium are stable. 64Valence electrons in Gadolinium Gd10 65Valence electrons in Terbium Tb11 66Valence electrons in Dysprosium Dy12 67Valence electrons in Holmium Ho13 68Valence electrons in Erbium Er14 69Valence electrons in Thulium Tm15 70Valence electrons in Ytterbium Yb16 71Valence electrons in Lutetium Lu3 Lanthanum La 57.
Valence electrons are the sum total. See also what are the roles of producers consumers. A valence electron can either absorb.
A valence electron can exist in the inner shell of a transition metal. Determine group period and block from the given electron configuration. Atoms with 8 valence electrons or 2 in the case of helium are stable.
Most transition metals have an electron configuration that is ns2 n1d so those ns2 electrons are the valence electrons. Noble Gases The Group 18 elements are the noble gases. Some key characteristics of a valence electron are.
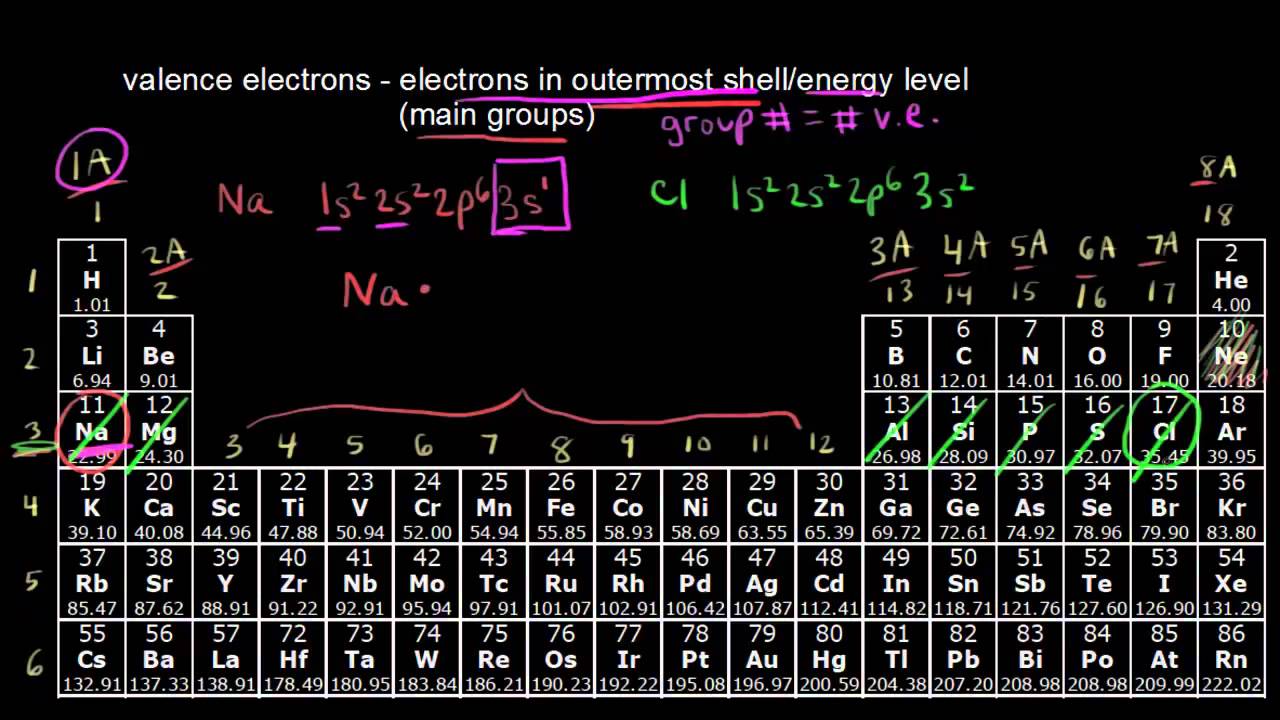
Counting Valence Electrons For Main Group Elements Video Khan Academy
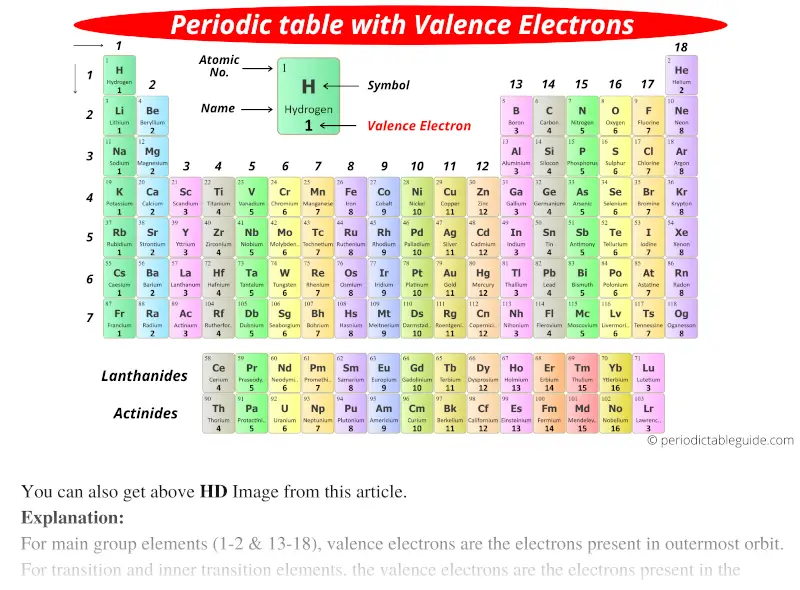
Periodic Table With Valence Electrons Labeled 7 Hd Images

What Are Valence Electrons Chemtalk

How To Find The Number Of Valence Electrons For Transition Metals Youtube

Transition Metal Ion Formation Chemistry For Non Majors

How To Find Valence Electrons Chemistry Classroom Science Chemistry Chemistry

How To Find The Number Of Valence Electrons Using A Periodic Table Electrons Energy Level Chemistry
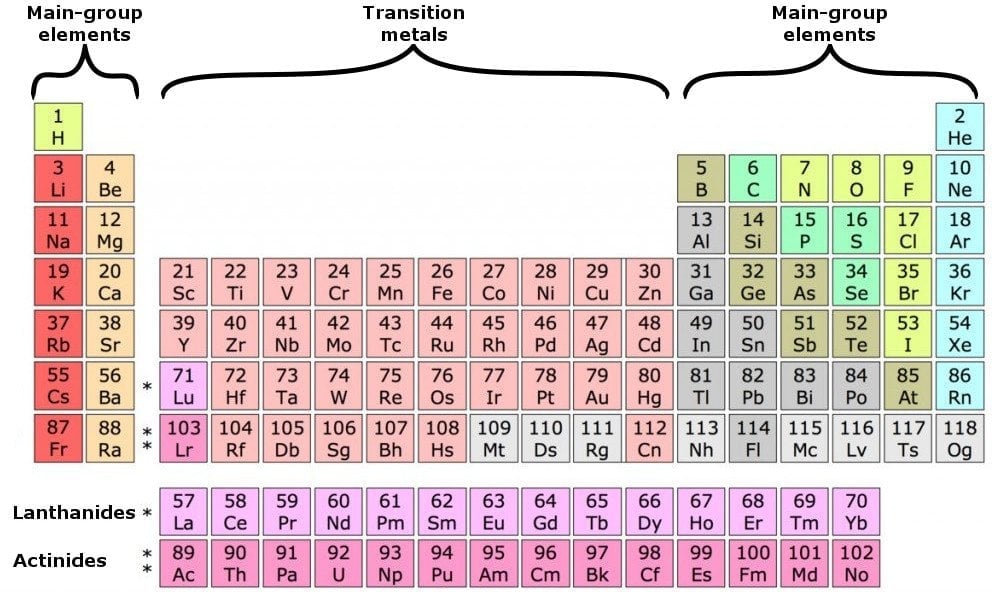
What Are Valence Electrons And How To Find Them Where Are They Located

Comments
Post a Comment